Shopping cart
Your cart empty!
Terms of use dolor sit amet consectetur, adipisicing elit. Recusandae provident ullam aperiam quo ad non corrupti sit vel quam repellat ipsa quod sed, repellendus adipisci, ducimus ea modi odio assumenda.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Sequi, cum esse possimus officiis amet ea voluptatibus libero! Dolorum assumenda esse, deserunt ipsum ad iusto! Praesentium error nobis tenetur at, quis nostrum facere excepturi architecto totam.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore, soluta alias eaque modi ipsum sint iusto fugiat vero velit rerum.
Sequi, cum esse possimus officiis amet ea voluptatibus libero! Dolorum assumenda esse, deserunt ipsum ad iusto! Praesentium error nobis tenetur at, quis nostrum facere excepturi architecto totam.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore, soluta alias eaque modi ipsum sint iusto fugiat vero velit rerum.
Dolor sit amet consectetur adipisicing elit. Sequi, cum esse possimus officiis amet ea voluptatibus libero! Dolorum assumenda esse, deserunt ipsum ad iusto! Praesentium error nobis tenetur at, quis nostrum facere excepturi architecto totam.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore, soluta alias eaque modi ipsum sint iusto fugiat vero velit rerum.
Sit amet consectetur adipisicing elit. Sequi, cum esse possimus officiis amet ea voluptatibus libero! Dolorum assumenda esse, deserunt ipsum ad iusto! Praesentium error nobis tenetur at, quis nostrum facere excepturi architecto totam.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore, soluta alias eaque modi ipsum sint iusto fugiat vero velit rerum.
Do you agree to our terms? Sign up
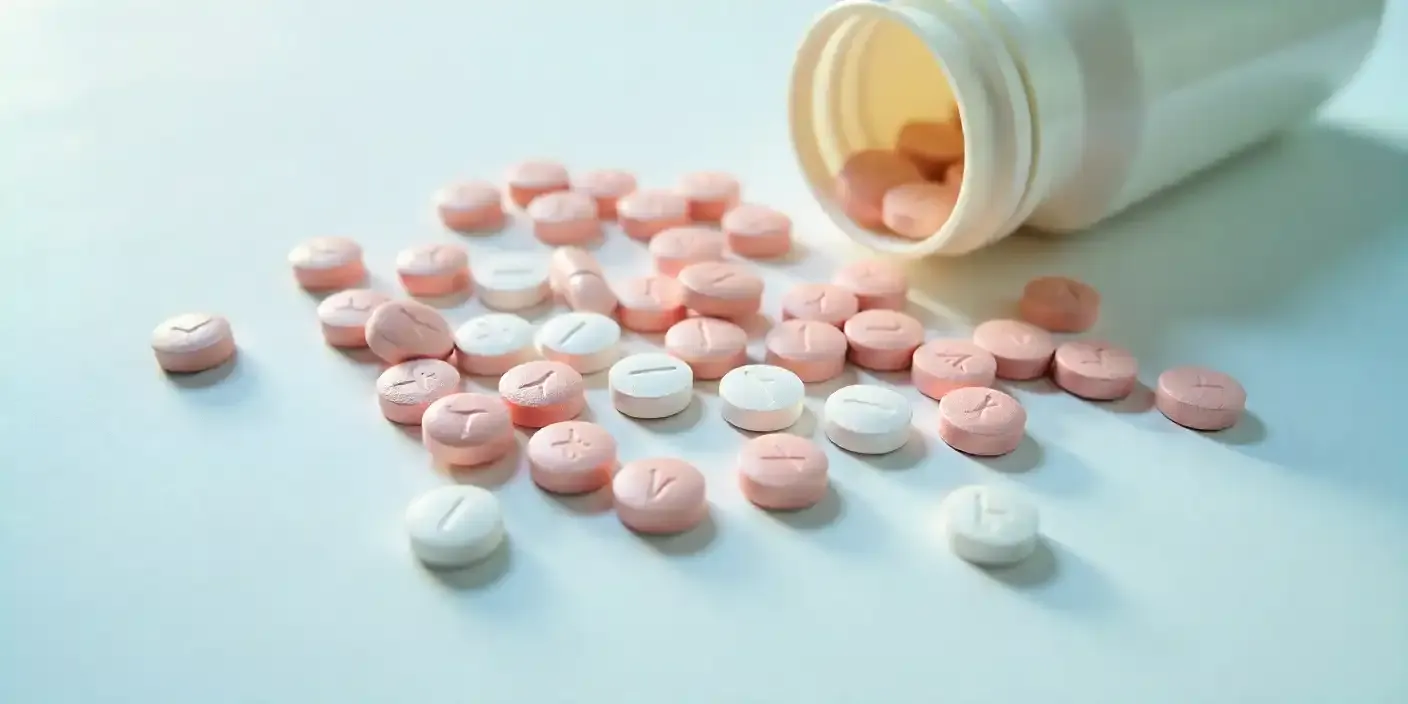
A statewide probe in Rajasthan has revealed a major lapse in drug regulation, exposing the circulation of substandard and counterfeit medicines that endangered thousands of lives. The discovery comes amid ongoing concerns over deaths linked to contaminated cough syrup.
Documents reviewed by India Today TV show that over the past year, hundreds of drug samples failed quality checks. These include essential antibiotics, anti-allergics, painkillers, anti-diabetic medicines, and heart drugs. Alarmingly, many of these drugs were already sold in bulk before test results flagged them as substandard.
Among the failed medicines:
Antibiotics: Amoxicillin, Clavulanic Acid, Ciprofloxacin, Cefpodoxime, Ceftriaxone – over 1 lakh units sold from Medirich Ltd.
Steroids: Betamethasone – three batches failed; over 30,000 tablets sold by Medivel Biotic.
Anti-allergics: Levocetirizine, Montelukast – 35,000 units from Therawin Formulation.
Anti-diabetics: Glimepiride, Pioglitazone – 18,000 units sold by Relief Biotech.
Painkillers & supplements: Aceclofenac, Paracetamol, calcium, vitamin D3 – more than 60,000 doses sold.
Heart drugs: Losartan – 10,000 tablets from Amex Pharma failed quality tests.
Officials warn that these numbers reflect only the visible part of a much larger crisis, with some drugs missing active ingredients or being contaminated, including injectable fluids meant for hospital use.
Rajasthan’s Drug Controller Commissioner, T Shubhmangalam, announced intensive inspections of 65 pharmaceutical manufacturers over the coming days. He emphasized, “We are taking substandard drugs very seriously.”
The crisis is compounded by weak enforcement. Under law, every failed drug sample should trigger prosecution, yet many cases remained pending. Former Drug Controller Rajaram Sharma, suspended for allegedly protecting offending companies, failed to send samples for national blacklisting, delaying action.
Current Drug Controller Ajay Phatak said, “23 counterfeit drugs were found in 2023, 29 in 2024, and three this year. Court permission has been received for 16 prosecutions, while interstate cases are still pending.”
Experts warn that counterfeit or substandard drugs are widespread in India, with outdated laws, weak enforcement, and jurisdictional confusion allowing millions to consume unsafe medicines before regulators can act.
26
Published: Oct 08, 2025