Shopping cart
Your cart empty!
Terms of use dolor sit amet consectetur, adipisicing elit. Recusandae provident ullam aperiam quo ad non corrupti sit vel quam repellat ipsa quod sed, repellendus adipisci, ducimus ea modi odio assumenda.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Sequi, cum esse possimus officiis amet ea voluptatibus libero! Dolorum assumenda esse, deserunt ipsum ad iusto! Praesentium error nobis tenetur at, quis nostrum facere excepturi architecto totam.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore, soluta alias eaque modi ipsum sint iusto fugiat vero velit rerum.
Sequi, cum esse possimus officiis amet ea voluptatibus libero! Dolorum assumenda esse, deserunt ipsum ad iusto! Praesentium error nobis tenetur at, quis nostrum facere excepturi architecto totam.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore, soluta alias eaque modi ipsum sint iusto fugiat vero velit rerum.
Dolor sit amet consectetur adipisicing elit. Sequi, cum esse possimus officiis amet ea voluptatibus libero! Dolorum assumenda esse, deserunt ipsum ad iusto! Praesentium error nobis tenetur at, quis nostrum facere excepturi architecto totam.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore, soluta alias eaque modi ipsum sint iusto fugiat vero velit rerum.
Sit amet consectetur adipisicing elit. Sequi, cum esse possimus officiis amet ea voluptatibus libero! Dolorum assumenda esse, deserunt ipsum ad iusto! Praesentium error nobis tenetur at, quis nostrum facere excepturi architecto totam.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore, soluta alias eaque modi ipsum sint iusto fugiat vero velit rerum.
Do you agree to our terms? Sign up
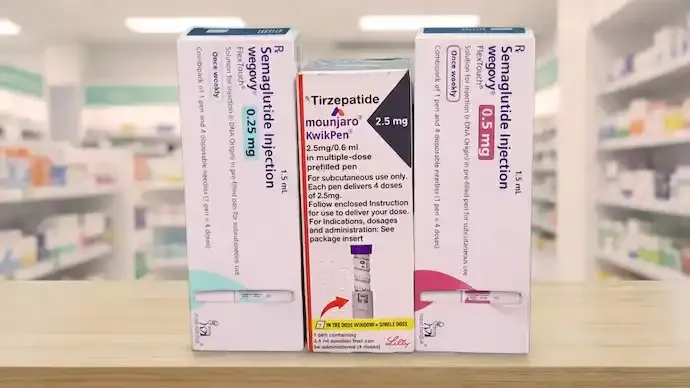
India is witnessing a rapid surge in demand for new-generation weight-loss medications, raising concerns among health experts and regulators about misuse, self-prescription, and gaps in enforcement of drug dispensing rules. Originally developed to treat type 2 diabetes, drugs containing semaglutide and tirzepatide are increasingly being used for weight management, reflecting growing public anxiety around obesity and metabolic disorders.
India already faces a dual health burden, with over 10 crore people living with abnormal blood sugar levels and an estimated 25 crore classified as obese. Against this backdrop, the introduction of globally popular anti-obesity drugs such as Mounjaro and Wegovy has triggered unprecedented demand, despite their high cost and classification as prescription-only medicines.
Medical experts warn that the rising popularity of these medications is being driven in part by self-prescription and easy access. Although Indian regulations require a valid prescription for all medicines, enforcement remains inconsistent. Consumers frequently obtain prescription drugs without medical documentation from neighbourhood pharmacies or through online platforms.
The situation may intensify further as semaglutide is set to go off-patent in India, potentially reducing prices by up to 70 percent when generic versions enter the market. At the same time, global developments such as oral versions of these therapies could expand accessibility, increasing pressure on regulatory oversight.
Both Mounjaro and Wegovy are designed to be administered as weekly injections under medical supervision. They are approved for use only when prescribed by specialists and are intended to complement structured diet and lifestyle changes. However, reports indicate that the medicines can be obtained quickly from pharmacies and e-pharmacy platforms with minimal verification procedures.
The financial barrier remains significant, with a month’s starter dose costing over ₹10,000. Despite this, demand continues to rise, particularly in metropolitan markets. Pharmaceutical companies have partnered with domestic manufacturers to expand distribution into smaller cities, targeting India’s rapidly growing anti-obesity market, which is projected to grow substantially by 2030.
These drugs work by mimicking gut hormones that regulate appetite and blood sugar. Tirzepatide acts on dual hormone receptors, while semaglutide targets GLP-1 receptors, helping reduce hunger and improve metabolic control. While generally considered safe under medical supervision, they can cause side effects such as nausea, vomiting, bloating, and diarrhoea. In rare cases, more serious complications may occur, reinforcing the importance of professional guidance.
Medical specialists caution that these therapies should be prescribed only for individuals meeting clinical criteria, such as a body mass index (BMI) above 30, or above 27 with associated health conditions. Experts warn that unsupervised use, cosmetic weight-loss goals, or incorrect dosing may increase health risks and undermine treatment effectiveness.
Pharmaceutical manufacturers maintain that these medications are prescription-only therapies intended for supervised use. Industry representatives emphasize patient safety, proper clinical evaluation, and responsible dispensing practices.
The rapid expansion of weight-loss drug use highlights the need for stronger enforcement, public awareness, and medical oversight. Without improved regulatory vigilance, experts warn that easy access to powerful metabolic drugs could lead to misuse, adverse health outcomes, and long-term public health consequences.
4
Published: 2h ago